Abstract
Venetoclax (BCL2 inhibitor) based regimens have significantly improved outcomes for elderly patients with acute myeloid leukemia (AML). While most patients initially respond to venetoclax-based regimens, upfront resistance or relapse on therapy occurs in many.
Therefore, elucidating the mechanistic link between BCL2 inhibition and AML cell death is crucial to finding novel therapeutic strategies to combat venetoclax resistance and to discover predictors of venetoclax response.
Previous studies have shown BCL2 has a non-canonical function in modulating oxidative phosphorylation (OXPHOS) activity of AML stem cells (LSCs). However, the mechanistic link between BCL2 and OXPHOS has not yet been established. BCL2 has been shown to regulate intracellular calcium localization by modulating the activity of calcium channels at the ER and mitochondria. Optimal mitochondrial calcium concentrations are crucial for cell metabolism as calcium overload or decrease can inhibit OXPHOS activity.
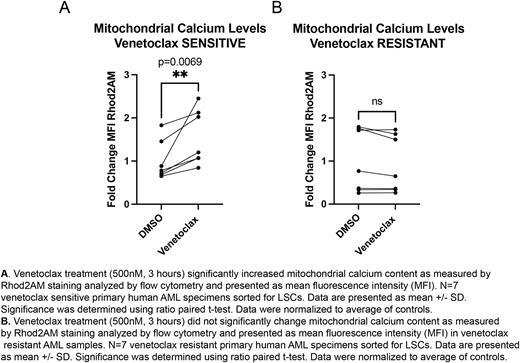
In preliminary studies, we established venetoclax increases mitochondrial calcium levels (Fig. 1A) leading to a subsequent decrease in OXPHOS activity and changes in the activity of key calcium dependent TCA cycle enzymes in primary human AML LSCs. Genetic inhibition of BCL2 in LSCs phenocopied the effects of venetoclax treatment. Based on these data, we hypothesized that BCL2 exerts its effect on LSC metabolism by modulating calcium localization between the ER and mitochondria. Furthermore, we sought to explore whether dysregulation of calcium localization by venetoclax is a mechanistic component of drug activity that leads to suppression of OXPHOS.
To determine how BCL2 may be influencing mitochondrial calcium levels, we assessed the relationship between BCL2 and SERCA3, a key ion channel responsible for calcium uptake into the ER. Venetoclax disrupted the physical interaction between BCL2 and SERCA3. Mechanistically, venetoclax perturbed this interaction by decreasing SERCA3 protein levels. These findings were recapitulated by genetic inhibition of BCL2 in LSCs. Functional analysis of SERCA3 via siRNA-mediated knock-down in primary AML specimens demonstrated increased mitochondrial calcium levels, decreased OXPHOS activity and decreased engraftment of immune deficient NSG-S mice. Based on these data, we propose a model in which venetoclax disrupts SERCA function leading to less calcium uptake at the ER and more calcium sequestered into the mitochondria leading to overload, decreased OXPHOS activity and decreased LSC function.
Next, we evaluated how calcium pathways were impacted in venetoclax resistant AML patient specimens. Intriguingly, venetoclax did not affect mitochondrial calcium levels (Fig. 1B) or the relationship between BCL2 and SERCA3. Therefore, we tested whether inhibiting the activity of MCU, the channel responsible for calcium uptake into the mitochondrial matrix, would target venetoclax resistant LSCs as it was overexpressed in this population. MCU inhibition led to decreased mitochondrial calcium levels, decreased OXPHOS activity and decreased LSC engraftment. These data indicate that venetoclax-resistant AML cells are no longer subject to changes in calcium localization induced by drug treatment but do retain an overall reliance on calcium regulation for LSC function/survival.
Finally, we assessed whether our findings could be leveraged to predict venetoclax sensitivity. Analysis of single cell RNA-seq data from venetoclax sensitive versus resistant AML patient specimens (n=19) revealed multiple calcium pathway gene sets were enriched in venetoclax resistant LSCs. Additionally, levels of certain genes including SERCA3 and MCU were differentially expressed in primitive versus monocytic clusters between sensitive and resistant specimens.
Taken together, our data demonstrate an unexpected and critical link between BCL2 and calcium signaling in human LSCs. A surprising aspect of our work is that venetoclax, a BH3 mimetic, acts to modulate the interaction with SERCA3. Lastly, our findings suggest that targeting intracellular calcium dynamics may represent a novel therapeutic strategy in the context of venetoclax resistant AML. In addition, we show that there are significant differences in calcium gene signatures between venetoclax sensitive vs resistant AML specimens that may be useful in predicting patient response to venetoclax based regimens.
Disclosures
Engel:Kura Oncology: Research Funding; Syros Pharmaceuticals: Research Funding; Argenx: Research Funding. Gillen:Elevate Bio: Research Funding; Kura Oncology: Research Funding; Syros Pharmaceuticals: Research Funding; Argenx: Research Funding. Ransom:Kura Oncology: Research Funding; Syros Pharmaceuticals: Research Funding; Argenx: Research Funding. Staggs:Kura Oncology: Research Funding; Elevate Bio: Research Funding; Syros Pharmaceuticals: Research Funding; Argenx: Research Funding. Smith:Syros: Research Funding; AML JV: Research Funding; Kura: Research Funding; Argenx: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal